
Place To Dip A Quill Crossword Clue, NYT Crossword Solve 2021-08-17 Tuesday, 23.92 MB, 17:25, 0, NYT Crossword over Lunch, 2021-08-17T07:20:08.000000Z, 8, Place to dip a quill crossword clue - CrosswordUniversal.com, crossworduniversal.com, 510 x 292, jpeg, quill, 20, place-to-dip-a-quill-crossword-clue, KAMPION
Which of the following is not a colligative property of solution? Osmotic pressure 1 see answer advertisement advertisement ot4blink ot4blink letter a is the answer. Thus, solubility is the answer. Colligative properties are not dependent on the chemical nature of the solution’s components.
We can observe the colligative properties of solutions by going through the following. Which of the following is not a colligative property of a solution? When a 51. 31 ml sample of h 2 so4 is titrated with 0. 7859 m naoh, 28. 88 ml of naoh solution is required to neutralize the h 2 so 4. what is the molarity of the h 2 so 4?. Those properties of ideal solutions which depend only on the number of particles of the solute (molecules or ions) dissolved in a definite amount of the solvent and do not depend on the nature of solute are called colligative properties. Tho important colligative properties are (i) relative lowering of vapour pressure. Which of the following is not a colligative property ? A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Which of the following is not a colligative property

Which of the following is NOT a colligative property | Chegg.com

Solved: Which Of The Following Is NOT A Colligative Proper... | Chegg.com

Which one of the following is not a colligative property?

Which of the following is a not a colligative | Chegg.com

Solved: Which Of The Following Is A Not A Colligative Prop... | Chegg.com
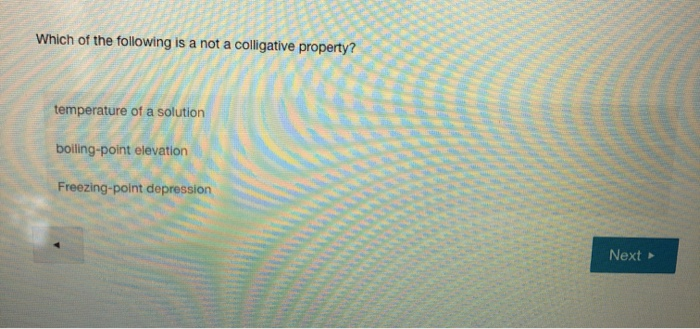
Solved: 13. Which Of The Following Is Not A Colligative Pr... | Chegg.com

OneClass: Which of the following is a not a colligative property? a

Solved: Student Name: 1. Which One Of The Following Is NOT... | Chegg.com

Solved: Which Of The Following Is Not A Colligative Proper... | Chegg.com
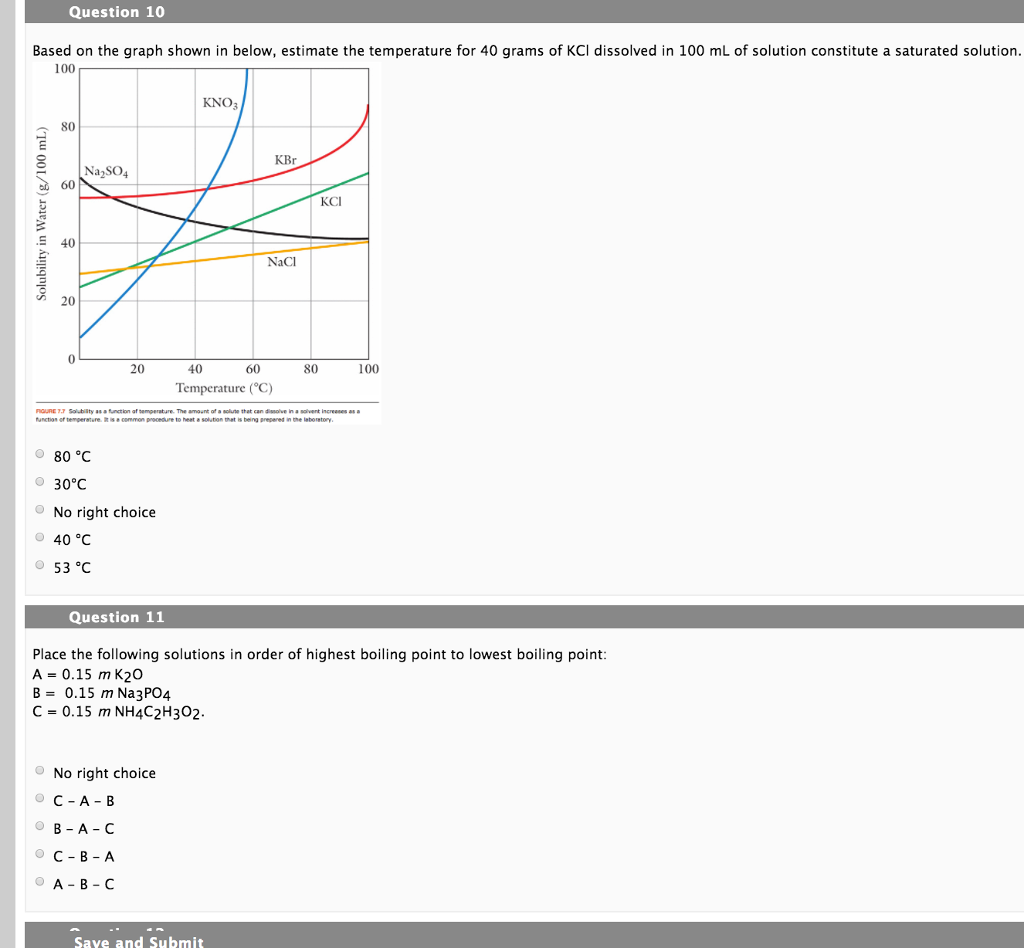
.JPG)
EmoticonEmoticon