
Place To Dip A Quill Crossword Clue, NYT Crossword Solve 2021-08-17 Tuesday, 23.92 MB, 17:25, 0, NYT Crossword over Lunch, 2021-08-17T07:20:08.000000Z, 8, Place to dip a quill crossword clue - CrosswordUniversal.com, crossworduniversal.com, 510 x 292, jpeg, quill, 20, place-to-dip-a-quill-crossword-clue, KAMPION
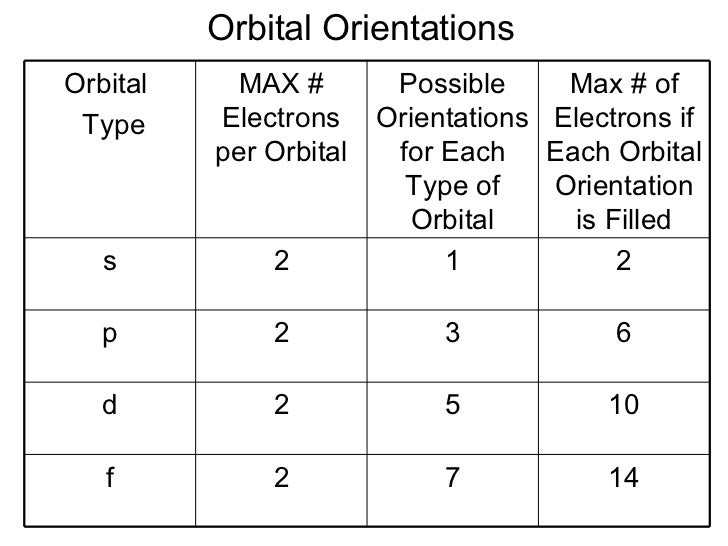
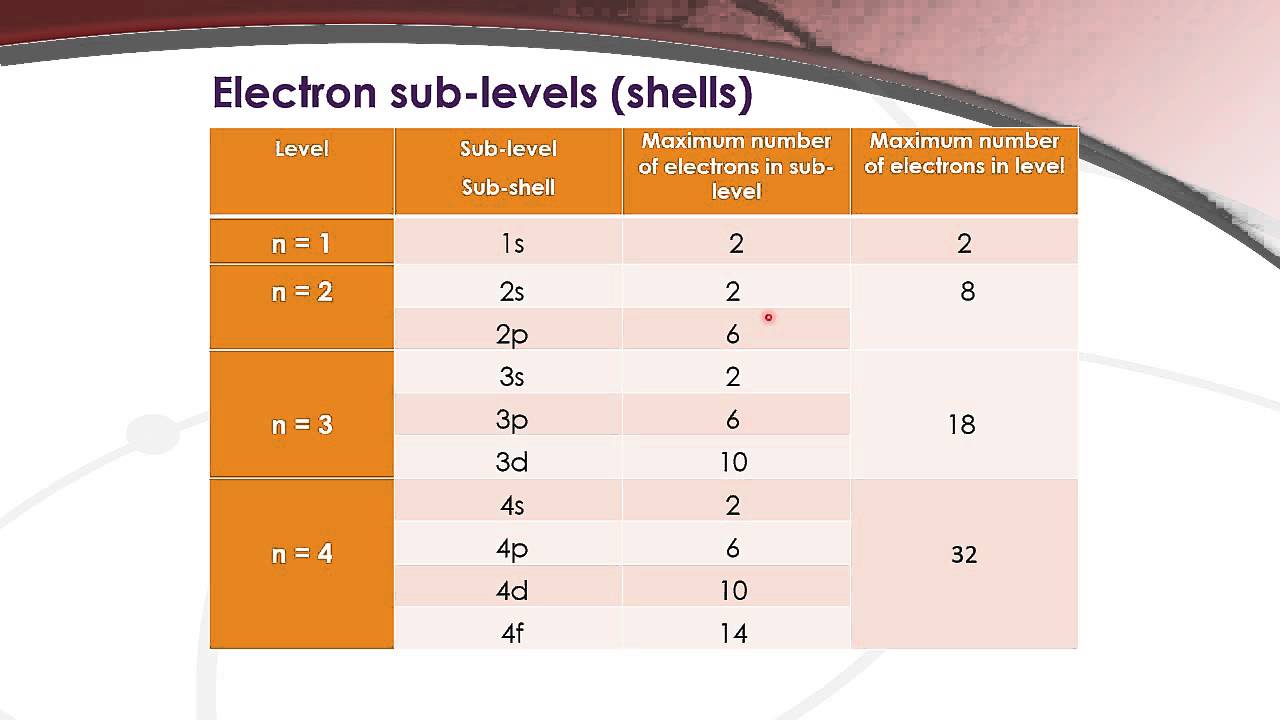
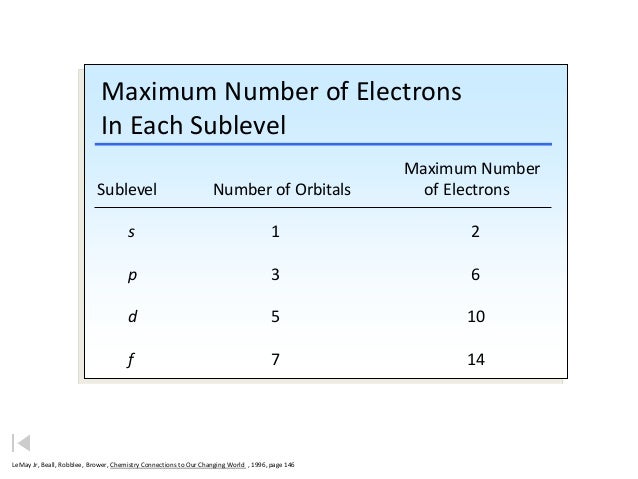
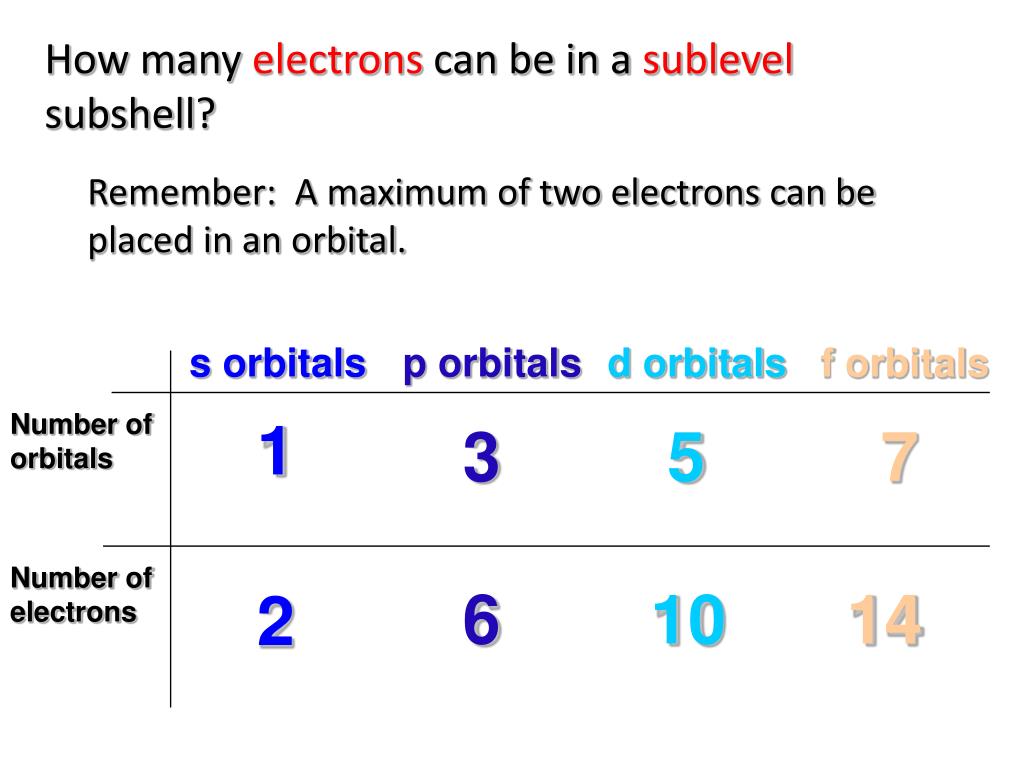
How many electrons can an orbital of type f hold? However, i got it wrong and the correct answer is marked as c (2). According to hund’s rule of maximum multiplicity, electrons are dispersed throughout the orbitals of a subshell in such a way that the maximum number of unpaired electrons and electrons with the same spin direction is obtained. Faqs on filling of electrons in orbitals.
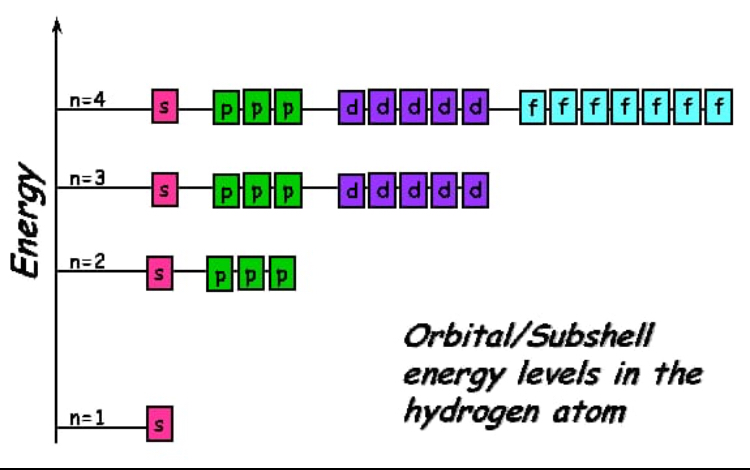
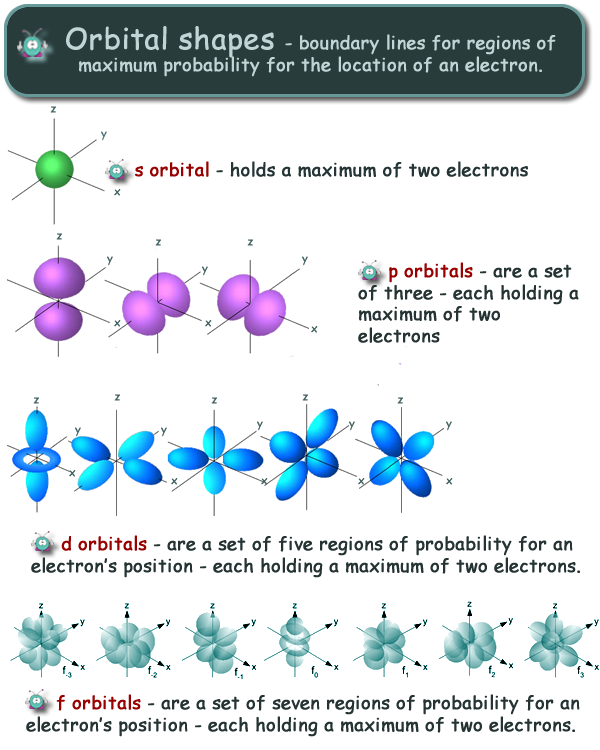
Orbitals are the place where the electrons live or where they are found. The subshells which include s, p, d, and f the contain the orbitals. The numbers are as follows; The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max. And the 4 sublevel has 7 orbitals, so can contain 14 electrons max. The p sublevels are called 2p, 3p, and 4p. How many electrons are in the 4p sublevel? As each orbital can hold maximum two electrons so total electrons.
Orbitals

Unit3presentation
Maximum Number Of Electrons In An Orbital - How Many Queens Are In A
Electron config
Physics For Foundation: chapter 2-matter (part 1)

Magnetic Quantum Number: Definition & Example - Video & Lesson
PPT - Quantum Mechanical Model of the Atom PowerPoint Presentation
Electrons | Facts, Summary & Definition | Chemistry Revision
Physics revision | GCSE and A Level Physics Revision | Cyberphysics
EmoticonEmoticon