
Place To Dip A Quill Crossword Clue, NYT Crossword Solve 2021-08-17 Tuesday, 23.92 MB, 17:25, 0, NYT Crossword over Lunch, 2021-08-17T07:20:08.000000Z, 8, Place to dip a quill crossword clue - CrosswordUniversal.com, crossworduniversal.com, 510 x 292, jpeg, quill, 20, place-to-dip-a-quill-crossword-clue, KAMPION
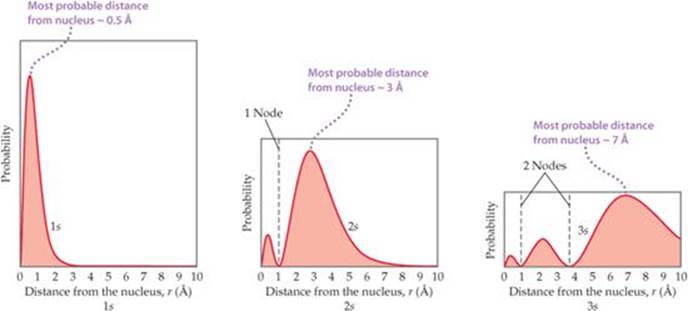
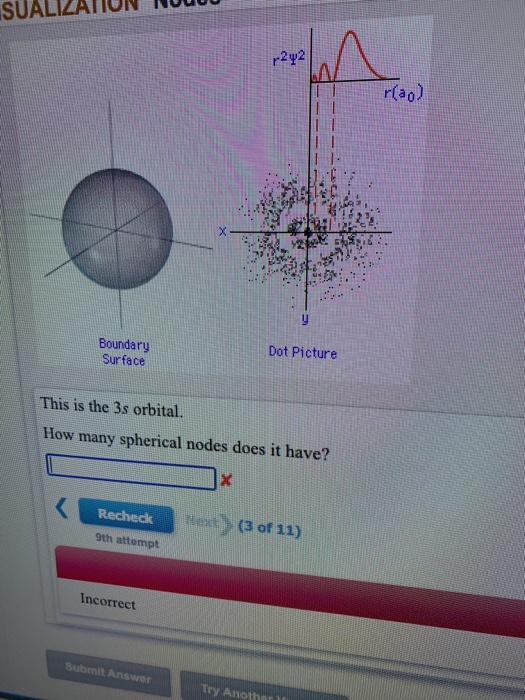
Here ‘l’ denotes azimuthal quantum number and ’n’ denotes principal quantum number. In 3s orbital there are zero nodal planes ,as it is spherical in shape. If you want angular node in this orbital ,which is equ. How many radial nodes are present in 3s orbital.
2 radial nodes are present in 3s orbital. To know in detail about atomic structure, visit byju’s. 4. 5 (1) (4) (2) choose an option that best describes your problem. Answer not in detail. There are two spherical nodes in 3s orbital. Reason there is no planar node in 3s orbital. View solution > what are the numbers of nodes present in: 1. 1s 2. 2s 3. 2p Then, how many radial nodes are present in 3s orbital?
atoms - What are the physical manifestations of radial nodes

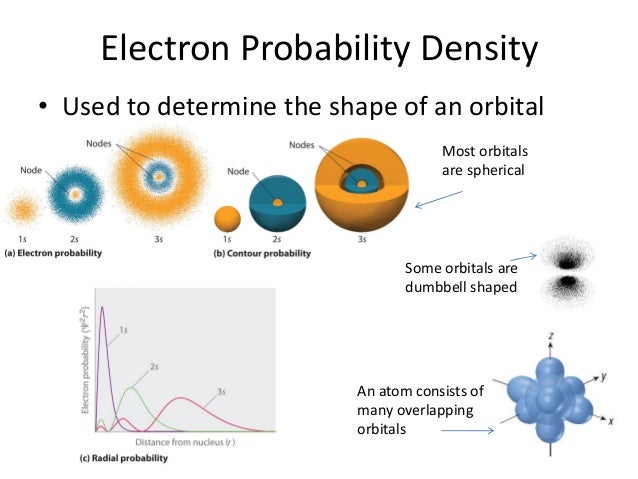
Comparing the radial probability distributions for the 1 s , 2 s , and
Solved: Su P242 Rau) X Boundary Surface Dot Picture This I... | Chegg.com
Radial probability distributions for the 1... | Clutch Prep

33 thequantummechanicalmodeloftheatom
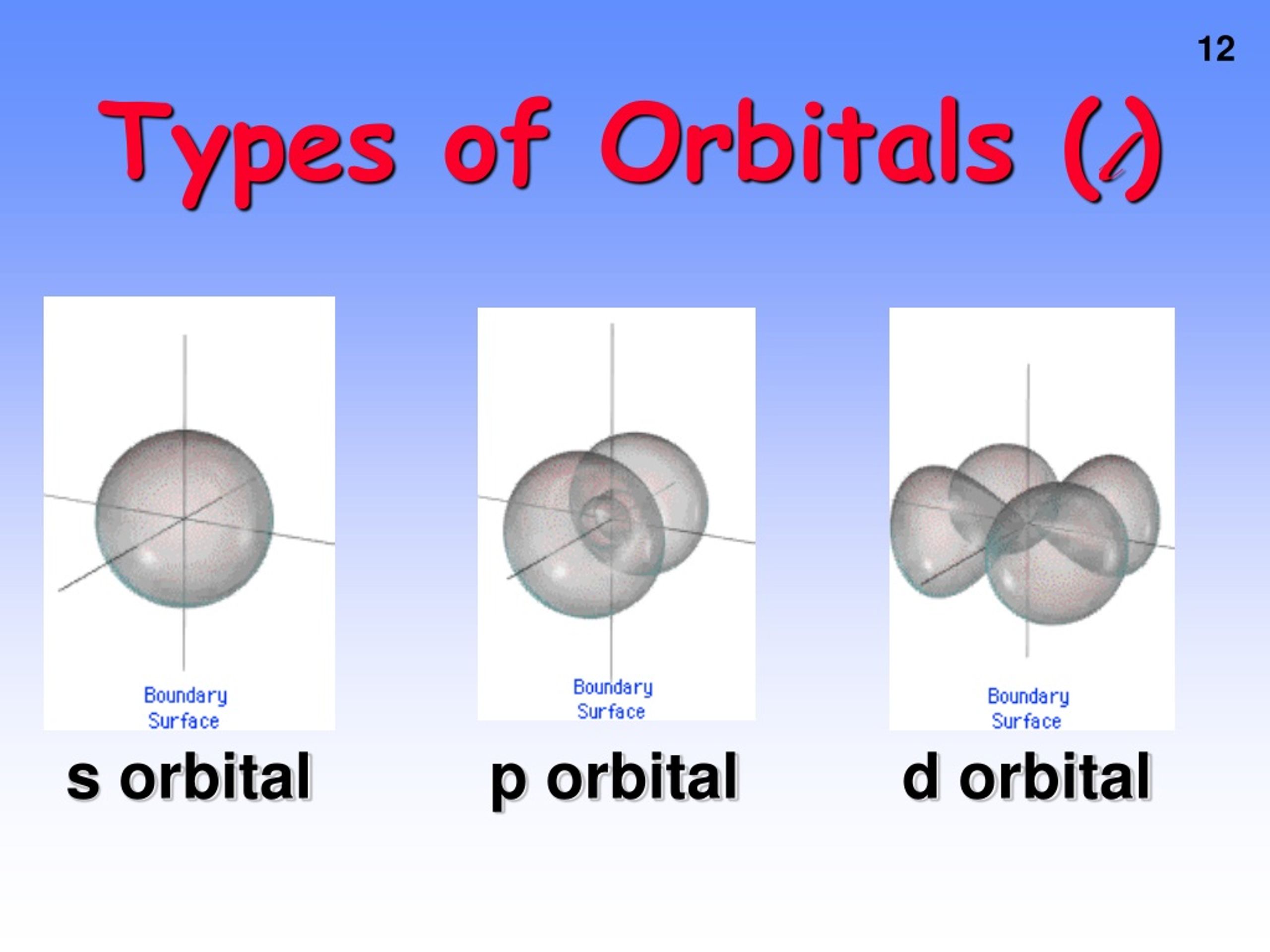
physical chemistry - What are the maximum number of electrons in each

PPT - Electron Configurations PowerPoint Presentation, free download
Semiconductor Physics and Devices : December 2013

Number of radial nodes orbitals in 3s and 2p - Brainly.in
In the plots of radial distribution function for the hydrogen 3s

EmoticonEmoticon