Place To Dip A Quill Crossword Clue, NYT Crossword Solve 2021-08-17 Tuesday, 23.92 MB, 17:25, 0, NYT Crossword over Lunch, 2021-08-17T07:20:08.000000Z, 8, Place to dip a quill crossword clue - CrosswordUniversal.com, crossworduniversal.com, 510 x 292, jpeg, quill, 20, place-to-dip-a-quill-crossword-clue, KAMPION
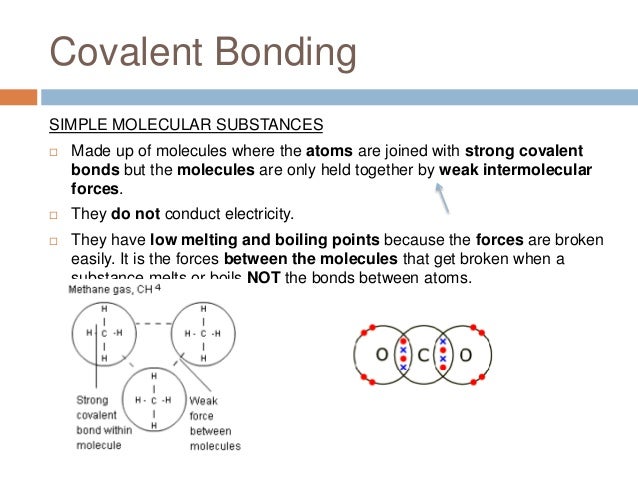
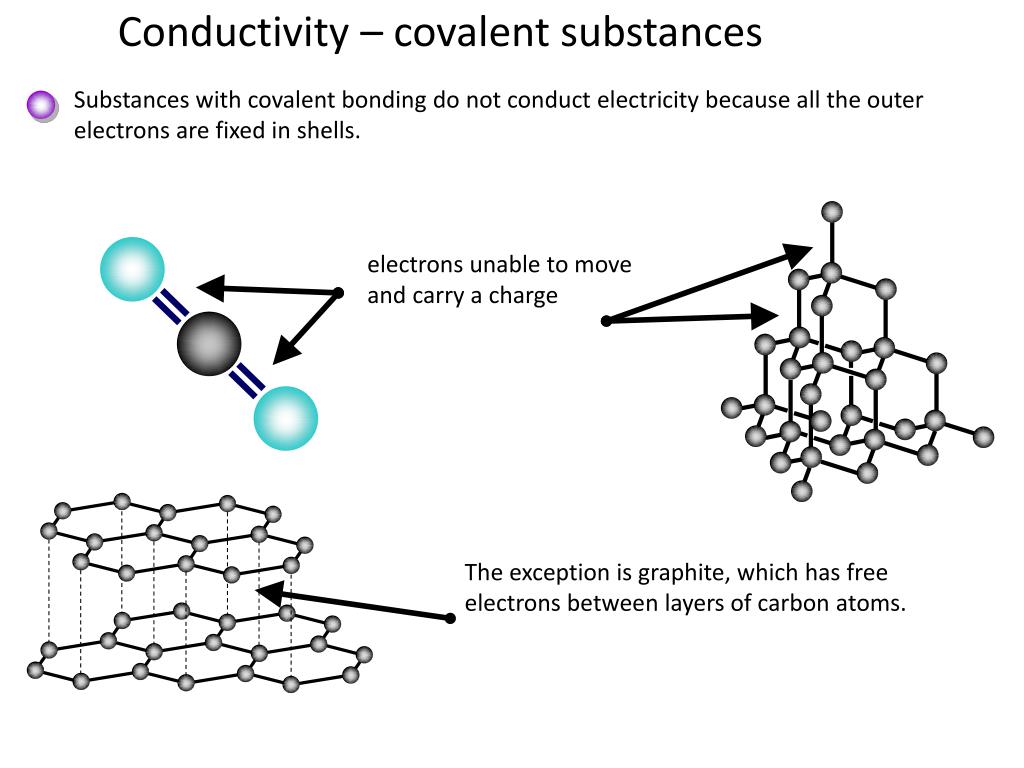
However, covalent molecular compounds do not conduct electricity when they are melted because they do not transfer electrons unless they react. Moreover, ionic crystals are much harder than molten solids and can be split by pressing against each other. Why do covalent compounds conduct electricity? Covalent compounds do not conduct electrical.
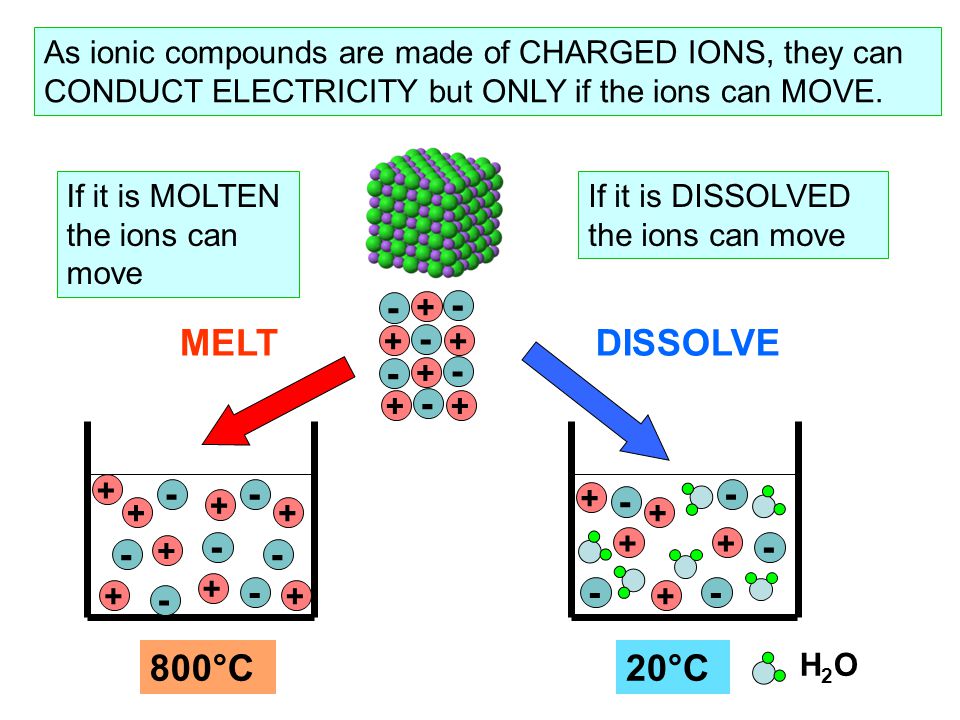
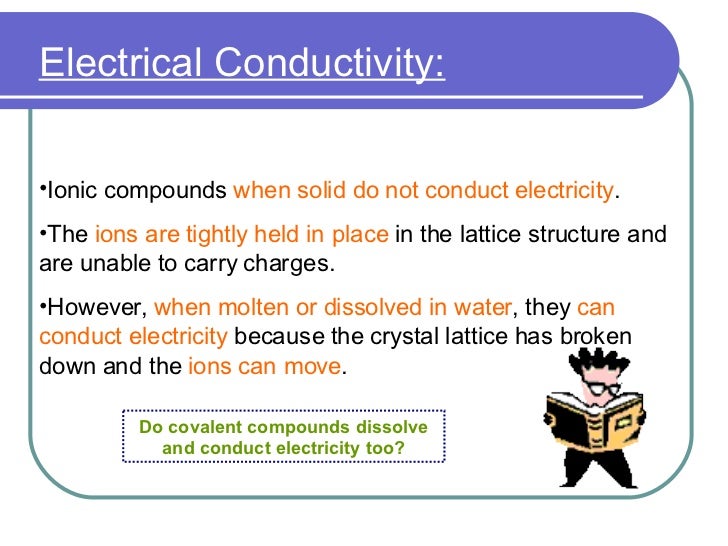
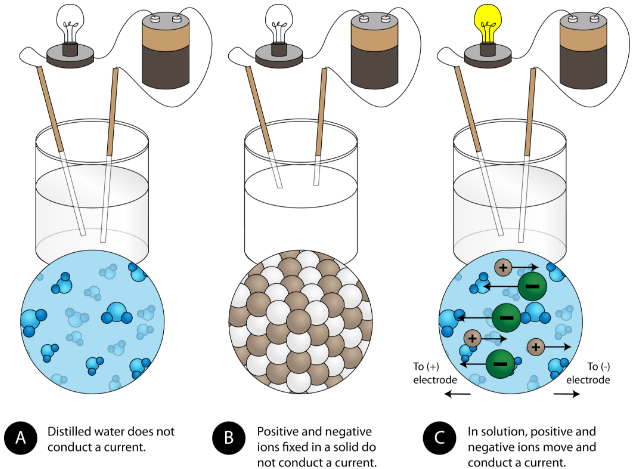
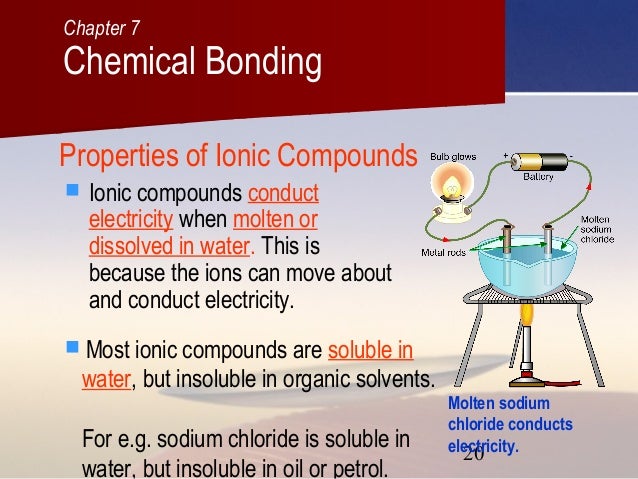
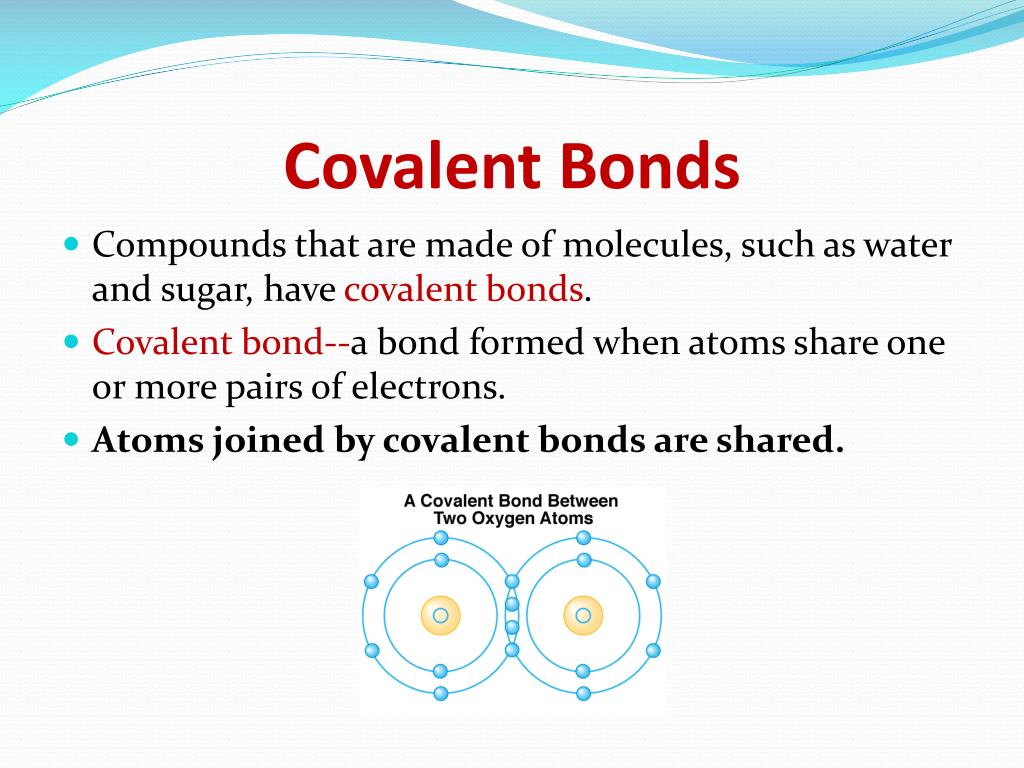
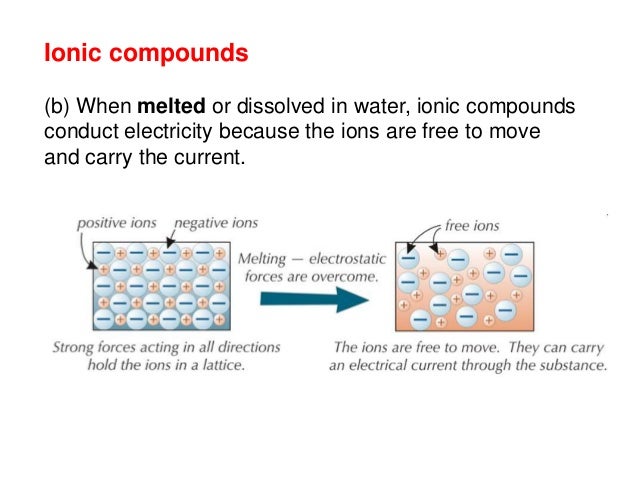
In case of ur question the method must be conduction and for conduction they require some material or say s. These compounds are now counted in thousands, and they are extremely important in the country's economy however, in molten state,they become good conductors of electricity when molten, however, it can conduct electricity because its ions are able to move freely through the ionic compounds dissolve in the presence of water because the positive. This is very simple. Ionic compounds are the ones which furnish ions in the aqueous solution, and hence helps in the conduction of electricity. Covalent compounds are the ones which do not furnish ions in the solution. Covalent compounds (exception of graphite) do not conduct electricity because all electrons are tightly held between the atoms, so they are not free to move around and continue an electric current. 1:42 understand why compounds with giant ionic lattices have high melting and boiling points; 1:43 know that ionic compounds do not conduct electricity when solid, but do conduct electricity when molten and in aqueous solution (g) covalent bonding. 1:44 know that a covalent bond is formed between atoms by the sharing of a pair of electrons
How can you use electrical conductivity to decide if a compound is

Gcse chemistry unit 2a
Properties of Compounds - Ionic, Covalent and Metallic
8.9: Physical Properties of Ionic Compounds - Chemistry LibreTexts
C07 chemical bonding
PPT - The Structure of Matter Chapter 6 PowerPoint Presentation, free
PPT - Properties of Substances PowerPoint Presentation, free download
C2.2 how structure influences
Chem matters ch7_covalent_bonding

EmoticonEmoticon