Is Al(oh)3 Soluble In Water

Place To Dip A Quill Crossword Clue, NYT Crossword Solve 2021-08-17 Tuesday, 23.92 MB, 17:25, 0, NYT Crossword over Lunch, 2021-08-17T07:20:08.000000Z, 8, Place to dip a quill crossword clue - CrosswordUniversal.com, crossworduniversal.com, 510 x 292, jpeg, quill, 20, place-to-dip-a-quill-crossword-clue, KAMPION
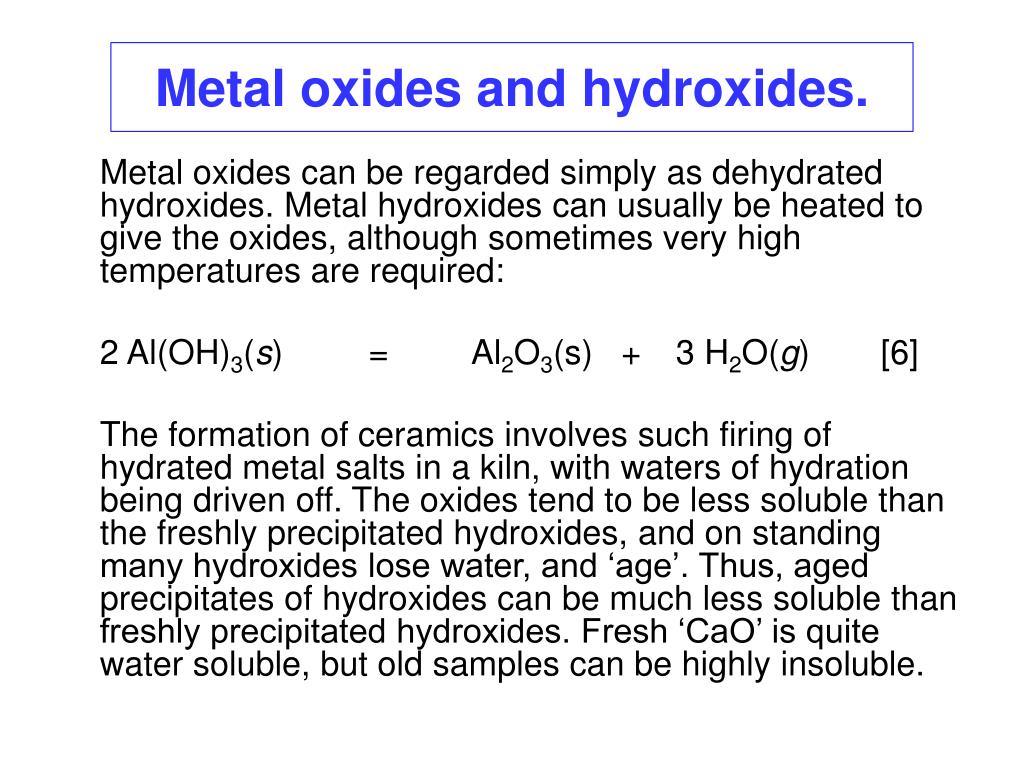
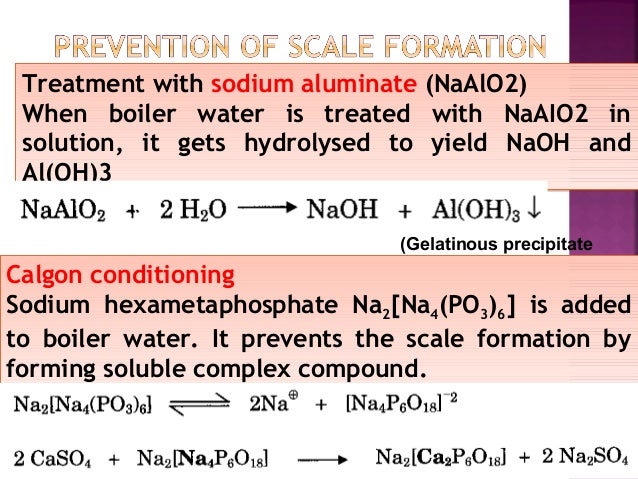
Al (oh)3 ( aluminium hydroxide ) is insoluble in water. What is soluble and insoluble ? Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of.
The attraction of water or aluminum, three plus and o. I cannot overcome the lattice energy, and aluminum hydroxide is insoluble in water. Um, for sodium hydroxide, the hydration energy is large eyes large enough to overcome the smaller a lot of energy and sodium hydroxide his soluble in water. In order for a salt to the soluble, theirs ions must be stable. That means, that cations should have a low polarization power and anions should not be very polarizable. The both magnitudes are very closely related. Other forms include aloh 2 + (aq) en al(oh) 3 (aq). In what way and in what form does aluminum react with water? [al(h 2 o) 6] 3+ (aq) solubility of aluminum and aluminum compounds.
Is Al(OH)3 Soluble or Insoluble in Water? - YouTube

Is Al Oh 3 Soluble In Water - Water Ionizer
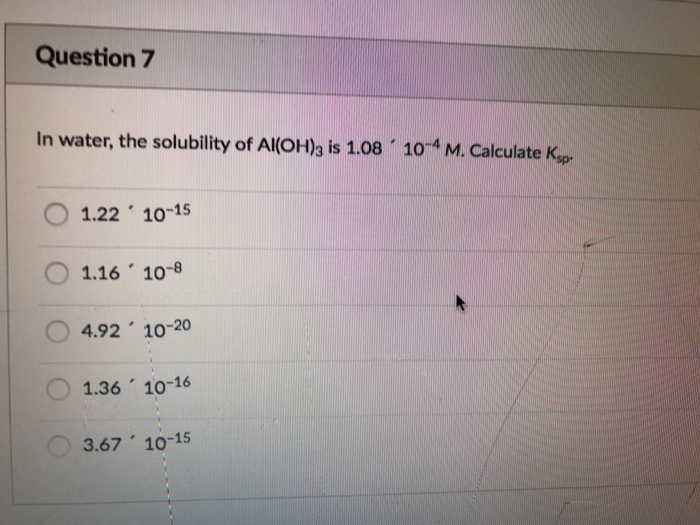
Solved: In Water, The Solubility Of Al(OH)_3 Is 1.08 ' 10^... | Chegg.com
Is Al Oh 3 Soluble In Water - Water Ionizer
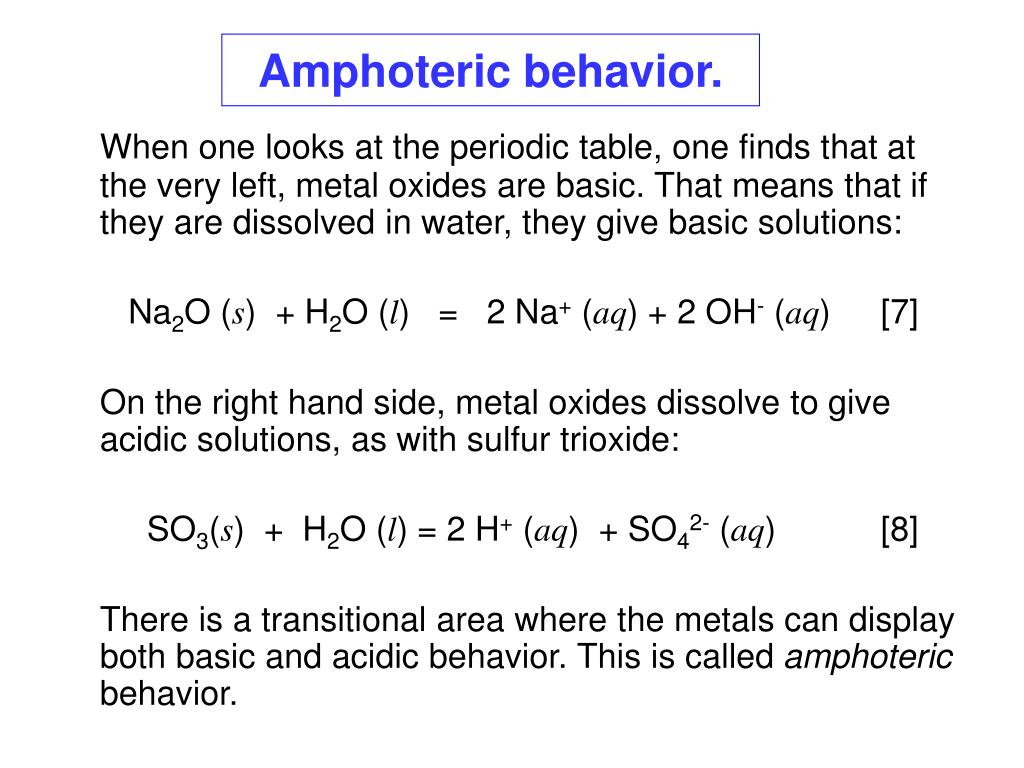
PPT - Solubility of metal hydroxides, and amphoteric behavior
solubility of Mg(OH)2 in pure water is 9 57 * 10^-3 grms/lit find its

(PDF) Al(OH)3 Facilitated Synthesis of Water-soluble, Magnetic

PPT - Solubility of metal hydroxides, and amphoteric behavior
Water
What is the molar solubility of fe(oh)3 in pure water? (ksp for fe(oh)3